

How to citeGómez, A. S et a l., (2024). Face Recognition and Cultural Variability: Analysis of Empirical Evidence and Evaluation Measures. Ánfora, 31 (57), 299 - 325 . https://doi.org/10.30854/anf.v31.n57.2024.1102 |
Anyerson Stiths Gómez-Tabares2 https://orcid.org/0000-0001-7389-3178 CvLAC https://scienti.minciencias.gov.co/cvlac/visualizador/generarCurriculoCv.do?cod_rh=0001583946 Colombia
Jainer Amézquita Londoño3 https://orcid.org/0000-0002-7844-6753 CvLAC https://scienti.minciencias.gov.co/cvlac/visualizador/generarCurriculoCv.do?cod_rh=0001482946 Colombia
David Antonio Pineda Salazar4 https://orcid.org/0000-0002-1080-4796 CvLAC https://scienti.minciencias.gov.co/cvlac/visualizador/generarCurriculoCv.do?cod_rh=0000045640 Colombia
|
Objective: To review scientific literature in which controlled cognitive tasks and recording of neurological activity are utilized to evaluate face recognition ability, considering the "other-race effect" (ORE). Methodology: Reflection article, whose methodology is based on a literature review; 15 studies were included for meta-synthesis. Results: It was found that subjective-recollection controlled cognitive tasks, electroencephalography technique, and event-related potentials predominate in face recognition research, considering the ORE. It was also found that oxytocin has no influence on face memory, and that difficulties in recognizing other-race blurred faces correlate with the activation of the fusiform face area (FFA). Conclusions: Neural processing of other-race faces requires more effort, evidenced by larger N250 amplitude, and it is related to N170 component. Furthermore, own-race face recognition is prolonged when these are inverted. of Other-race face processing may be increased by instruction, whereas anger does not improve other-race face memory. This review confirms that both neurophysiology and cultural factors play a crucial role in face recognition and suggests that ORE may be produced by the interaction between these factors.
Keywords: face recognition; other-race effect (ORE); controlled cognitive tasks; electroencephalography (EEG); cultural neuroscience (obtained from the thesaurus DeCS/MeSH – Health Science Descriptors).
Objetivo: revisar la literatura científica que utiliza tareas cognitivas controladas y registro de actividad neurológica para evaluar la capacidad para reconocer rostros, considerando el «efecto de la otra raza» (EOR). Metodología: artículo de reflexión, cuya metodología parte de una revisión de la literatura; se incluyeron 15 estudios para la meta-síntesis. Resultados: se encontró que predominan las tareas cognitivas controladas de recuerdo subjetivo y técnica de electroencefalografía, y potenciales relacionados con eventos en la investigación sobre el reconocimiento de rostros, considerando el EOR. Se halló que la oxitocina no influye en la memoria facial, y que las dificultades en reconocer caras borrosas de otras razas correlacionan con la activación del área fusiforme facial (AFF). Conclusiones: el procesamiento neuronal de rostros de otras razas requiere más esfuerzo, evidenciado por mayor amplitud del componente N250, y relacionado con la N170. Además, invertir rostros de la propia raza prolonga su reconocimiento. La instrucción puede incrementar el procesamiento de caras de otras razas, mientras que la ira no mejora su memoria facial. Esta revisión confirma que tanto la neurofisiología como los factores culturales juegan un papel crucial en el reconocimiento facial, y sugiere que el EOR puede ser un producto de la interacción entre estos factores.
Palabras clave: reconocimiento facial; efecto de la otra raza (EOR); tareas cognitivas controladas; electroencefalografía (EEG); neurociencia cultural (obtenidos del tesauro DeCS/MeSH Descriptores en Ciencias de la Salud).
Objetivo: rever a literatura científica que utiliza tarefas cognitivas controladas e registro da atividade neurológica para avaliar a capacidade de reconhecer rostos, considerando o "efeito de outra raça" (EOR). Metodologia: artigo de reflexão, cuja metodologia parte de uma revisão da literatura; foram incluídos 15 estudos para a metassíntese. Resultados: foi descoberto que predominam as tarefas cognitivas controladas de lembrança subjetiva e a técnica de eletroencefalografia, e potenciais relacionados a eventos na pesquisa sobre o reconhecimento de rostos, considerando o EOR. Verificou-se que a oxitocina não influencia a memória facial e que as dificuldades em reconhecer rostos borrados de outras raças correlacionam-se com a ativação da área fusiforme facial (AFF). Conclusões: o processamento neuronal de rostos de outras raças requer mais esforço, evidenciado por uma maior amplitude do componente N250 e relacionado ao N170. Além disso, inverter rostos da própria raça prolonga seu reconhecimento. A instrução pode aumentar o processamento de rostos de outras raças, enquanto a raiva não melhora sua memória facial. Esta revisão confirma que tanto a neurofisiologia quanto os fatores culturais desempenham um papel crucial no reconhecimento facial e sugere que o EOR pode ser um produto da interação entre esses fatores.
Palavras-chave: reconhecimento facial; efeito de outra raça (EOR); tarefas cognitivas controladas; eletroencefalografia (EEG); neurociência cultural (obtidos do tesauro DeCS/MeSH - Descritores em Ciências da Saúde).
Faces are visual stimuli that convey perceptual and social information (Schwartz et al., 2023; Shoham et al., 2022). Perceptual information is relevant since it allows for facial features recognition; for example, nose size, mouth size or eye color. It also allows for automatic social inferences, which are consistent with the perceived attributes, to be made (Abudarham & Yovel, 2016; Schwartz & Yovel, 2019a, 2019b; Shoham et al., 2022).
Face recognition is a complex neurocognitive process linked to visual processing and social encoding, as well as to the segmentation of perceived facial features and attributes, and the integration of these to construct a coherent and unique representation of a person's face (Blais et al., 2021; Chua et al., 2005; Schwartz et al., 2023; Tarr & Gauthier, 2000). Face recognition also involves the identification and representation of specific facial features and their integration into each individual’s unique mental model (Blais et al., 2021).
Face recognition and facial expression processing are different, but related, sharing cognitive processes and some brain mechanisms and pathways (Duchaine & Yovel, 2015; Yamamoto et al., 2020). The essential difference between them is that face recognition is more associated with registering facial features, while facial expression processing is associated with the interpretation of socio-emotional aspects (Yamamoto et al., 2020).
On the one hand, face recognition begins with the perception of basic facial features, such as shape and texture, which are processed by the primary and secondary visual brain areas. These features are then integrated into a more complex face representation in the inferior temporal cortex (ITC), which includes the fusiform facial area (FFA) (Kanwisher et al., 1997; Kanwisher & Yovel, 2006; Pitcher & Ungerleider, 2021; Sellal, 2022). Finally, this facial representation is compared with mental models stored in memory to identify the person (Haxby et al., 2000; Lopatin et al., 2018).
On the other hand, facial expression processing implies the ability to process social and affective information based on the facial expression (Bigelow et al., 2022; Shoham et al., 2022; Zhen et al., 2013). It involves the activity of the posterior superior temporal sulcus (PSTS) as well as the limbic system (Atkinson & Adolphs, 2011; Duchaine & Yovel, 2015; Haist & Anzures, 2017). In this sense, face recognition and facial expression processing capabilities are essential for the success of social interactions, their modulation, and communication with others throughout life.
In studies with functional magnetic resonance imaging (fMRI), three central regions associated with face recognition have been identified in the occipito-temporal cortex: FFA, PSTS, and the region of the inferior occipital gyrus (IOG) (Gobbini & Haxby, 2007; Karimi-Rouzbahani et al., 2021; Pitcher et al., 2014; Sellal, 2022; Zhen et al., 2013). These three regions constitute the core system for face recognition. The FFA and the PSTS region process distinctive facial features, such as gaze direction, lip movements, and facial expression. The IOG region is responsible for processing invariant aspects that underlie the recognition of individuals (Zhen et al., 2013). Additionally, it has been reported that the amygdaloid nucleus and the insula are involved in the processing of emotional stimuli of facial expressions (Furl et al., 2013; Gobbini & Haxby, 2007; Pitcher et al., 2014; Sellal, 2022).
Zhen et al. (2013) and Sellal (2022) maintain that face recognition is a hierarchical and efficient process that involves multiple neural networks specialized in different aspects of face recognition. The process begins in primary visual areas and progresses to more specialized areas. A main pathway connects the occipital cortex, where the occipital facial area (OFA) is located, to the fusiform facial area (FFA) in the fusiform gyrus, which plays a critical role in recognizing facial identity and its invariant aspects. The second sub-network connects the left middle frontal gyrus (LMFG) and the inferior frontal gyrus (IFG), related to accessing semantic information gleaned from faces; such as a person’s name and biographical information (Zhen et al., 2013).
The third sub-network includes regions associated with social facial perception, such as gaze movement and orientation, facial expressions, and lip movements. This network extends from the primary visual cortex to the superior temporal sulcus (STS), and it is known as the third pathway of visual recognition (Sellal, 2022; Pitcher & Ungerleider, 2021; Shoham et al., 2022). It also involves the PSTS, the orbitofrontal cortex (OFC), and the insular cortex (IC), which are especially linked to facial expression. Furthermore, additional functional systems, such as the intraparietal sulcus (responsible for the management of spatial attention), the primary auditory cortex (prelexical speech perception), and the limbic system (emotion perception), are connected to the core system of visual processing for face recognition (Sellal, 2022).
The role of the posterior superior temporal sulcus (PSTS) in the processing of visual stimuli linked to movement, facial expressions, and gaze (Pitcher & Ungerleider, 2021; Pitcher et al., 2020; Sliwinska et al., 2020), and in processes that support social cognition, such as intentional attribution and theory of mind (Saxe & Kanwisher, 2003) has been confirmed by several studies. Thus, evidence supports a neurocognitive model oriented to the hierarchical and efficient processing that involves multiple specialized neural networks, in which facial visual processing begins with the identification of basic features and advances to more complex levels of mental representation processing (Haxby et al., 2000; Zhen et al., 2013; Sellal, 2022). However, several questions remain unanswered about the interaction between these areas and how they are affected by cultural and individual factors.
Research has shown that face recognition is not uniform across cultures and contexts, and that cultural variability can affect how the faces of individuals from different ethnic groups are processed and remembered (Liu et al., 2019; Kelly et al., 2007). This has led neuroscientists to explore the effect of cultural variability, such as race, gender, ethnicity, and in-group biases, on facial processing (Hugenberg et al., 2007, 2010; Liu et al., 2019).
This is a clear example is the “other-race effect” (ORE), which describes how people tend to be more accurate at recognizing faces of their own race than those of other races (Meissner & Brigham, 2001). This effect has been documented in recent studies (Schwartz et al., 2023; Stelter & Schweinberger, 2023).
Therefore, studying how cultural factors and individual differences generate a differential effect on the brain areas involved in face recognition including how these differences are integrated into a model that explains the interaction between different processing levels and functional areas (Haxby et al., 2000; Zhen et al., 2013; Sellal, 2022). Furthermore, investigating how these findings can contribute to a better understanding of brain plasticity, and the influence of sociocultural factors on visual processing is necessary. These aspects have important implications for the analysis of cognitive tasks and the value of the techniques that have been used in studies on face recognition, such as event-related potentials (ERPs), fMRI, and EEG.
In accordance with what has been stated so far, conducting a critical analysis of the evidence on the effect of cultural variability on facial processing and the cognitive tasks used in current scientific research is relevant. The objective of this work is to carry out a systematic review of scientific literature on face recognition and ORE, considering the controlled cognitive tasks used in neuroscientific research. In this review, the following aspects will be highlighted: available evidence on the differences in the cognitive and neural processes underlying face recognition according to racial social perception, controlled cognitive tasks, and the neuroimaging methods most commonly used for scientific research.
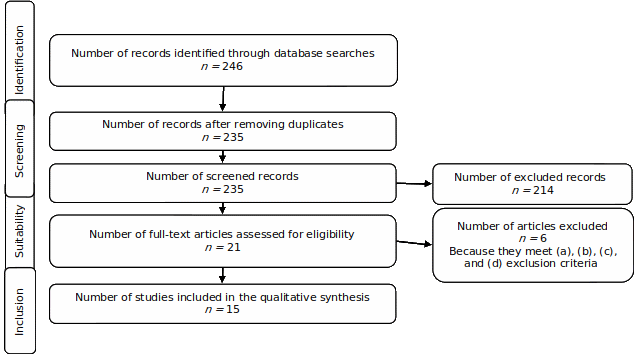
This is a reflective study, whose methodology is based on a literature review. PRISMA statement criteria were used for study eligibility (Page et al., 2021). The guidelines of this system are widely recognized in the scientific community and are used to ensure transparency and quality in the reporting of systematic reviews and meta-analyses. Database search process, eligibility criteria, and flow diagram are shown below.
Studies were identified by searching electronic databases: Scopus, Springer, and Science Direct. The search was conducted on April 23, 2023, with the following search strategy in English: "face recognition" AND "race" OR "ethnic" OR "culture" AND "eeg" OR "fmri" OR "ERPs". Only articles were chosen, for a total of 246 records. The search was performed by title, summary, and keywords, with no time restriction.
Taking into consideration that the objective of this study is to present the available evidence in neurosciences field, and the controlled cognitive tasks that are used to measure the effect of sociocultural factors on face recognition ability, the following inclusion criteria were defined: empirical experimental articles published in the last decade; (a) whose central topic is face recognition in humans and ORE; (b) in which brain functioning measures are used; (c) as well as controlled cognitive tasks. Excluded from this review were articles that: (a) have designs different from experimental research; (b) have a different approach to cultural variability and ORE; (c) are developed in samples with clinical or animal alterations; (d) make no clear presentation of the measurement paradigm of the cognitive task of face recognition; and (e) present no interest in ORE.
Each author of this study undertook the articles search in one of the databases and, when the articles to be included were identified, an ad-hoc table was created for them to register bibliometric information in it. After that, a conceptual identification of the paradigms on face recognition controlled cognitive tasks was carried out by grouping the tasks into categories.
This sample is documentary due to its systematic review design and poses no risk to humans. Additionally, respect for the sources and authors reviewed herein has been preserved.
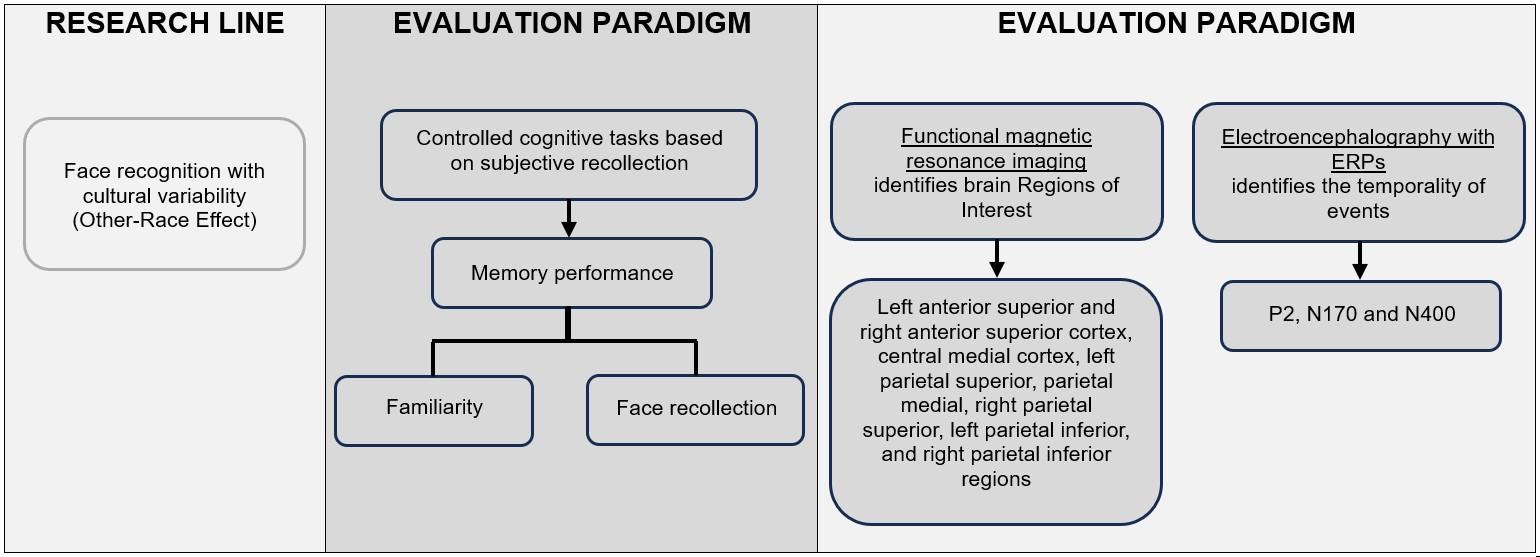
In the field of cognitive neuroscience, research on face recognition ability, considering cultural diversity, is a solid and defined research line that focuses on what was initially called "other-race bias," “cross-race effect" or "other-race effect" (ORE). This phenomenon has traditionally been studied through the use of cognitive task paradigms based on subjective recollection, with a specific focus on the evaluation of memory performance and two subprocesses that compose it: familiarity and recollection in regard to the face (Herzmann et al., 2013; Schwartz et al., 2023; Wong et al., 2021). This methodological framework facilitates the understanding of the nuances and dynamics of cognitive and neural processes involved in face recognition in different intercultural contexts.
Despite showing certain variations, the cognitive tasks applied in this research field generally involve the exposure to faces that are previously unknown to the experimental subject, followed by a learning phase in which a single image per face is presented. Subsequently, in the evaluation phase, these faces must be identified among a set of new distracting images (Tüttenberg & Wiese, 2019). The protocol is completed when participants make remember-know judgments about the viewed or observed faces (Herzmann et al., 2017).
Variations of this paradigm have been designed to examine perceptual and selective attentional processes in more detail, aiming to identify brain regions that are activated during face detection and to determine whether such activation is sensitive to specific facial components (features) or to a holistic representation of the face (configuration) (Zhao et al., 2014). In these adaptations of the paradigm, after each image is presented, the subject must make a judgment about the race of the face that appears in the image. These variants allow for a deeper analysis of cognitive and neural processes underlying face recognition in different cultural contexts.
Most neuroscientific research has explicitly or implicitly integrated a hierarchical explanatory model for facial processing in order to interpret accumulated empirical evidence. This model is based on the use of functional magnetic resonance imaging (fMRI) to identify the anatomical substrate corresponding to different hierarchical stages of facial processing, as well as on the use of electroencephalography (EEG), particularly event-related potentials (ERPs), to determine the precise temporality of these processes (Colombatto & McCarthy, 2017).
ERPs represent voltage fluctuations in specific segments of EEG signal, which, after filtering electromuscular activity (for example, that of the masseter and ocular muscles), allow for the visualization of electric fields associated with the activity in clusters of neurons. These fluctuations are manifested as oscillations, which are named based on the direction of the potential change (N1, N2 for negative upward deflections; P1, P2 for positive downward deflections) (Donchin, 1979). These patterns allow for a rigorous and detailed analysis of temporal and spatial dynamics of neural activity during facial processing.
Table 1 shows different functional imaging methods used along with face recognition controlled cognitive tasks in different populations. It also details aspects related to their resolution, application, advantages, and disadvantages.
Table 1. Functional Imaging Methods Used in Face Recognition Controlled Cognitive Tasks.
|
Imaging Method |
Resolution |
Application |
Advantages |
Disadvantages |
|
EEG |
Spatial - Low Temporal - High |
Study various rhythms, epilepsy, preoperative mapping, degenerative disorders. |
Non-invasive, no ionizing radiation, widely used, low cost. |
Low spatial resolution |
|
RM f |
Spatial - High Temporal - Low |
Preoperative mapping and functional mapping |
Non-invasive, can perform functional imaging |
High cost |
Adapted from: Brain Connectivity Analysis Methods for Better Understanding of Coupling (Shriram et al ., 2012).
In cognitive tasks execution, participants interact with various brain- activity recording technologies. These are selected based on the specific research objectives to identify Regions of Interest (ROI) activation, and to determine the precise moment of occurrence of specific processes. In the field of face recognition studies, ERPs occupy a prominent place, precisely because they have contributed to identifying three components strongly associated with differential perception of faces according to their ethnicity or race: P2, N170, and N400 (Yong et al., 2020). These components provide a detailed view of the temporality and characteristics of neural processing during face recognition, focusing specifically on the response to race/ethnicity-related variations.
Likewise, Table 2 shows the synthesis of studies included in this review, which highlights neurophysiological measures, controlled cognitive tasks and empirical evidence reported on face recognition ability, considering the ORE. Figure 2 shows an analytical synthesis diagram of the results presented herein.
Table 2 . Empirical Evidence on Face Recognition and ORE.
|
Authors (year) |
Sample |
Neurophysiological Measure |
Controlled Cognitive task |
Relevant Results |
|
(Herzmann et al ., 2013) |
52 young adults, half of them male ( M age 22.3, SD = 3.1) and the other half female ( M age 23.1, SD = 3.4). |
EEG recorded with 128-channel Geodesic Sensor Net. ERPs. |
Subjective recollection Remember-know judgment Yes/No No = familiar/unfamiliar |
Oxytocin did not affect memory for own-race and other-race faces when measured across all participants, nor did it have an effect when measured for females and males separately. Oxytocin did not differentially affect memory for female and male faces. It was shown that familiarity judgments with the faces studied are more accurate after oxytocin administration. |
|
(Zhao et al . , 2014) |
12 Chinese young adults, half of them females ( M age 23.7). |
f MRI performed on Philips Achieva 3.0T System . |
Identification of repeated presentation. |
Differences in own-race and other-race face recognition. Results regarding blurred faces were correlated with differences in FFA activation to those faces, suggesting that the processing configuration within the FFA may underlie the other-race effect in face recognition. |
|
(Herzmann, 2016) |
26 Caucasian young adults, 10 females ( M age 18, SD= 0.7). |
EEG recorded with 32-channel Easy- Cap TM . ERPs. |
Subjective recollection Remember-know judgment
1-4 recollection degree |
Increased N250 amplitudes for other-race faces are taken to represent higher neural demands on the identity-specific processing of other-race faces, which are generally processed less holistically, and less at the individual level. |
|
(Colombatto & McCarthy, 2017) |
31 Caucasian adults, 3 of them males ( M age 23.67, SD=4.8). |
EEG recorded with 64-channel Neuroscan Quik-Cap. ERPs. |
Repetition priming task Identifying race B/W |
Recognition of own-race faces takes longer if the face is inverted. Facial inversion revealed processing effects, involving areas of the pericalcarine extrastriate visual cortex and lateral occipito-temporal cortex. |
|
(Herzmann et al ., 2017) |
22 Caucasian young adults, 73% females ( M age 19.1 with SD = 1.4 years) and 12 East Asian (67% females ( M age 18.8 SD=1.1). |
EEG recorded with Easy- Cap TM . ERPs. |
Associative-memory task with subjective recollection of encoding moments.
Old / New Old > Blue / Orange |
First study with this type of task to evaluate face recognition. During the study phase, subsequently recognized other-race faces (with and without correct background information) elicited more positive mean amplitudes than own-race faces, suggesting higher neural activation during the encoding of other-race faces. |
|
(Wiese & Schweinberger, 2018) |
40 Caucasian adults, 20 males ( M age 23, SD = 2.7). |
EEG recorded with Biosemi Active II System. ERPs. |
Subjective recollection Remember-know judgment Old / New |
The own-race bias was accompanied by larger N170 responses to other-race faces, presumably reflecting more effortful perceptual processing of this facial category. |
|
(Herzmann et al ., 2018) |
36 Caucasian adults, 15 males ( M age 21 SD=2.5). |
EEG recorded with 128-channel Geodesic Sensor Net TM . ERPs. |
Facial inversion Subjective recollection Remember-know judgment 1-4 recollection degree |
First neural evidence that increased holistic processing during memory encoding contributes to the other-race effect in face memory. |
|
(Tüttenberg & Wiese, 2019) |
20 Caucasian adults, 10 females ( M age 23.6 SD=5.8). |
EEG recorded with 64-channel ANT Neuro System. ERPs. |
3 tasks Facial sorting Matching Object detection |
Better sorting and matching for own-race than for other-race identities was observed. |
|
(Proverbio et al ., 2020) |
24 Caucasian adults, 12 males ( M age 23.8, SD=4.23). |
128-channel EEG and EOG. ERPs. |
Subjective recollection Images of baby faces are included. Remember-know judgment Old / New |
A baby schema effect on N170, anterior N2, and P300 responses, which were larger to infant than adult faces, regardless of ethnicity. |
|
(Yong et al ., 2020) |
31 Asian adults, 20 females ( M age 23. 18 SD= 3.33). |
EEG recorded with 128-channel Geodesic Sensor Net TM . ERPs. |
Race identification by eye features: Caucasians/Asians |
A specific facial feature, the eyes region, can account for known effects of race perception on early brain potentials. |
|
(Tüttenberg & Wiese, 2021) |
36 Caucasian adults, 26 females ( M age 21.7 SD= 4.1). |
EEG recorded with 64-channel ANT Neuro System. ERPs. |
Subjective recollection Remember-know judgment Old / New |
Instructions increase analyses for other-race faces, suggesting that more processing resources are allocated to these faces during encoding. |
|
(Anzures & Mildort, 2021) |
52 white adults, 29 females ( M age 24.02 SD=2.04). |
EEG recorded with 64-channel Brain Vision recorder. ERPs. |
Subjective recollection Remember-know judgment Old / New |
Face recognition abilities and their interaction with implicit racial bias modulate the early stages of other-race facial processing. |
|
(Kacin & Herzmann, 2021) |
47 young adults, 27 Caucasian, (13 females, M age 19.7 SD= 1.36), 17 African American (13 females, M age 19.8, SD= 1.11). |
EEG recorded with Easy- Cap TM . ERPs. |
Subjective recollection First study with faces showing anger facial expressions to facilitate face recognition. Remember-know judgment Old / New |
Anger did not improve other-race facial memory in the behavior for either race of participants. It was evidenced that experience with same-race faces, and not stereotypes of other-race facial influences the ORE during memory retrieval. |
|
(Anzures et al ., 2022) |
18 Caucasian children, 13 females ( M age 6.53 SD= 0.79). 20 Caucasian children, 14 females ( M age 9.27, SD= 0.75), and 20 Caucasian adults, 14 females ( M age 19.74, SD= 1.86). |
EEG recorded with 64-channel Brain Vision recorder. (1.21.0303). ERPs. |
Subjective recollection
Remember-know judgment
Old / New |
Other-race faces elicited larger P100 amplitudes than own-race faces. Furthermore, adults with better other-race recognition proficiency showed larger P100 amplitude responses, compared to adults with worse other-race recognition proficiency. |
|
(Roth & Reynolds, 2022) |
46 10-month-old infants. |
EEG
recorded with
Geodesic
Sensor Net
TM
.
|
First study to analyze familiarization, attention and face recognition in infants. |
Infants at this age may process human faces more efficiently when familiarized with a single exemplar. |
The objective of this work was to conduct an intentional critical review of scientific literature on face recognition and ORE, considering the controlled cognitive tasks used in neuroscientific research. Several studies that analyzed the relationship between face recognition and cultural variability of ORE, often using neurophysiological measures and controlled cognitive tasks to explore these processes, were found (Anzures & Mildort, 2021; Anzures et al., 2022; Colombatto & McCarthy, 2017; Herzmann, 2016; Herzmann et al., 2013, 2017, 2018; Kacin & Herzmann, 2021; Proverbio et al., 2020; Roth & Reynolds, 2022; Tüttenberg & Wiese, 2019, 2021; Wiese & Schweinberger, 2018; Yong et al., 2020; Zhao et al., 2014).
The face recognition empirical studies reviewed herein, considering ORE, have focused attention on three key aspects. First, the stimuli used, which typically are high-resolution images of Caucasian, East Asian, and African American faces, previously unknown to the participants. Second, the use of specialized devices to record neuronal activity, such as fMRI and EEG with ERPs. The technical details of these devices, from brands and number of channels to data analysis strategies and electrode placement, are carefully described and controlled in these studies. The third element refers to the cognitive task development procedure, including the presentation of the images in blocks or sequences, the collection of participants' responses (generally through key pressing), and the instructions provided. Additionally, these studies often include additional measuring instruments, such as self-reports.
Controlled cognitive tasks used in face recognition evaluation stand out for the variations in the manipulation of face images used as stimuli, for example, in their orientation by using inverted faces (Colombatto & McCarthy, 2017) and images of the same face from different angles (Tüttenberg & Wiese, 2019). Likewise, variations are observed in their composition, by adding background colors in the encoding phase (Herzmann et al., 2017); in their sharpness, by presenting the images with a blurred effect (Zhao et al., 2014); in their structure, by showing only some facial features (Yong et al., 2020); or by adding facial expressions through faces with emotions such as anger (Kacin & Herzmann, 2021).
As distinctive characteristics presented in the sample section, the importance of reporting brain damage as well as the hand dominance of participants in the selection, indicating the differentiated execution precision in the use of computer keyboards in cognitive tasks, due to the effects of motor skills in neural measures recording, was observed in these studies (Colombatto & McCarthy, 2017; Herzmann, 2016; Herzmann et al., 2017; Kacin & Herzmann, 2021). This attention to detail reinforces the reliability and validity of the conclusions derived from these studies, reflecting the complexity of the neural and cultural processes involved in face recognition.
Neurophysiological research has shown a complex interrelationship of factors that influence face recognition when considering aspects such as ORE. In this regard, Herzmann et al. (2013) found that oxytocin did not differentially affect memory for other-race faces nor for male or female faces. Zhao et al. (2014) identified differences in brain activation related to own-race and other-race faces recognition. These studies laid the foundations for the work of Herzmann (2016), and that of Colombatto and McCarthy (2017), who showed that identity-specific processing and facial orientation are crucial in other-race face perception. For their part, Wiese and Schweinberger (2018) delved into these findings, demonstrating that neural responses can vary depending on the race of the perceived face.
Studies with EEG and ERPs indicated that neural response variations derived from other-race facial perception are concentrated especially in the left anterior superior and right anterior superior cortex, central medial cortex, left parietal superior, parietal medial, right parietal superior, left parietal inferior, and right parietal inferior regions. Recording neural activity in these areas has revealed that the frequencies that are most associated with the advantage in own-race face recognition, compared to that of other-race faces, are found in the N170, P2, and N400 potentials (Wiese & Schweinberger, 2018; Yong et al., 2020). From these, voltage maps based on difference waves in potentials that are evoked by visual stimulus between subsequent memory judgements, can be obtained. These maps show a window of between 500 and 900 ms for own-race and other-race facial recollection (Herzmann et al., 2018).
N170 is an ERPs recorded during face encoding between 150 and 190 ms. N170 is also a face-specific response that reflects processing of face schemata (Proverbio et al., 2020). P2, for its part, is usually related to early attentional processes that are often considered automatic, and which may reflect the activity of an early vigilance system dedicated to the detection of threat-related stimuli (Yong et al., 2020). Enlarged P200 responses reflect forceful processing that would elicit greater feedback from higher to lower visual areas (Anzures & Mildort, 2021). N400, recorded in 300–500 ms time window, measures facial familiarity processes; while the subsequent 500–800 ms recording reflects recollection processes (Herzmann et al., 2018; Rugg & Curran, 2007).
The study by Tüttenberg and Wiese (2019) showed that more processing resources tend to be allocated to own-race faces, resulting in better face sorting and recognition. These findings suggest learning advantages for own-race identities, and underscore the importance of perceptual experience in own-race bias. Furthermore, these findings are in agreement with what was reported by Herzmann et al. (2018), who noted that increased manipulations of holistic processing influence memory encoding for own-race compared to other-race faces. Studies in younger populations, such as that of Anzures et al. (2022), and that of Roth and Reynolds (2022), have added similar and complementary data by showing that children and infants show variations in other-race face perception.
The influence of neurophysiological and sociocultural factors interaction on face recognition is evidenced by the meticulous attention to stimuli, methodology, and demographic considerations in the studies reviewed herein (Anzures & Mildort, 2021; Anzures et al., 2022; Colombatto & McCarthy, 2017; Herzmann, 2016; Herzmann et al., 2013, 2017, 2018; Kacin & Herzmann, 2021; Proverbio et al., 2020; Roth & Reynolds, 2022; Tüttenberg & Wiese, 2019, 2021; Wiese & Schweinberger, 2018; Yong et al., 2020; Zhao et al., 2014). The relevance of ORE in neuroscience research is highlighted by these studies, which support the idea that differences in race perception can shape face recognition ability (Anzures et al., 2013; Ge et al., 2009; Kelly et al., 2007; Meissner & Brigham, 2001; Sangrigoli & de Schonen, 2004), and provide an interdisciplinary study perspective to analyze functional connectivity mechanisms for facial processing from a cross-cultural perspective (Wong et al., 2020).
Likewise, ORE has been shown to develop early during childhood (Anzures et al., 2022; Kelly et al., 2007; Roth & Reynolds, 2022; Sangrigoli & de Schonen, 2004), and is associated with limited exposure to other-race faces during critical development periods. This is due to the fact that, from birth, children have greater exposure to people belonging to their ethnic or racial in-group. Consequently, they develop a greater ability to identify and process the features and expressions of own-race faces, in addition to showing a preference for them compared to other-race faces (Bar-Haim et al., 2006; Hugenberg et al., 2007, 2010; Kelly et al., 2007).
According to the categorization-individuation model of the ORE (Hugenberg et al., 2007), in-group and out-group faces are attended in qualitatively different ways. In this regard, Prunty et al. (2023) maintain that identity-relevant features are attended more readily for in-group faces. This results in better performance in recognition memory tasks, while category-linked features are preferentially attended to in out-group faces, leading to faster categorization during search tasks. This results in an improved performance in recognition memory tasks, while category-related features are preferentially attended for out-group faces, which leads to an improved categorization speed in search tasks.
As children grow older, this preference becomes more pronounced, suggesting sociocognitive attitudes of implicit racial bias (Lebrecht et al., 2009). Early and frequent exposure to own-race faces promotes ORE during development (Anzures et al., 2013, 2022; Kelly et al., 2007). Factors such as intergroup contact, social exposure, formation of social stereotypes and in-group biases can strengthen the implicit racial biases of other-race face recognition (Anzures et al., 2022; Ge et al., 2009; Prunty et al., 2023). In this regard, Lebrecht et al. (2009) showed that perceptual other-race training (Caucasian people exposed to African American faces) reduced implicit racial bias, demonstrating its causal link with ORE. This effect has also been demonstrated in preschool children (Qian et al., 2019).
The above demonstrates the influence of exposure to specific sociocultural environments on neurocognitive processing of faces. These findings are consistent with what was reported in several of the studies in this review. Zhao et al. (2014) found a correlation between differences in FFA activation and recognition of other-race blurred faces, suggesting a neural basis of ORE. Herzmann (2016) observed that the presence of N250 amplitudes, increased for other-race faces, could indicate increased neural demands for identity-specific processing. This idea was supported by Wiese and Schweinberger (2018) as well as by Tüttenberg and Wiese (2021), who reported larger N170 responses for other-race faces, and an increase in analyzes for these faces due to specific instructions.
For their part, Colombatto and McCarthy (2017) and Herzmann et al. (2018) noted that recognition of own-race faces is slower when they are inverted, implying that increased holistic processing during memory encoding may contribute to ORE. Tüttenberg and Wiese (2019), as well as Anzures and Mildort (2021) expanded this finding by considering that high recognition abilities for own-race compared to other-race faces and identities are related to implicit racial biases. Finally, Anzures et al. (2022) provided evidence indicating that other-race facial perception generates larger P100 amplitudes than that of own-race faces; aspects that are associated with perceptual categorization processes and social perception.
Additional studies show that people have better face recognition memory for own- race compared to other-race faces (Liu et al., 2019; Zhou et al., 2021). Visual categorization of faces based on group membership (in this case, race) is thought to be a key component explaining changes in facial cognitive processing according to cultural membership (Hugenberg et al., 2010; Prunty et al., 2023). These findings have important implications for understanding the neuroscience of face processing in humans.
Data show that visual processing of faces can vary according to particular sociocultural exposure, which is associated with ORE. However, that there are universal neural mechanisms underlying face recognition (Blais et al., 2021; Caldara et al., 2010; Sellal, 2022; Zhen et al., 2013). This is consistent with previous studies where specific brain areas have been identified, such as the fusiform face area (FFA) and the Occipito-Temporal Sulcus (OTS) area, which are involved in other-race facial processing (Kanwisher et al., 1997; Karimi-Rouzbahani et al., 2021; Gauthier et al., 1999; Pitcher & Ungerleider, 2021; Sellal, 2022; Walker et al., 2008; Zhao et al., 2014).
Finally, ORE relates to cultural differences in visual information distribution and focus. It contributes to understanding how other-race faces are processed and perceived. These differences may have implications for how the neural mechanisms underlying face recognition adapt and adjust based on experience and cultural environment.
Studies on face recognition and ORE have used variations of cognitive tasks, which are controlled for measuring facial memory with EEG with ERPs preferential neurophysiological recording, which show greater interest in discovering process phases after having a solid theoretical foundation of ROI in FFA and OTS thanks to fMRI. These variations usually focus on stimuli presentation. Furthermore, neurophysiological activity recording focuses predominantly on the P100, N170, P2, and N400 potentials. In general, studies indicate better own-race than other-race face recognition.
Abudarham, N. & Yovel, G. (2016). Reverse Engineering the Face Space: Discovering the Critical Features for Face Identification. Journal of Vision, 16 (3), 1-18. https://doi.org/10.1167/16.3.40
Anzures, G. & Mildort, M. (2021). Do Perceptual Expertise and Implicit Racial Bias Predict Early Face-Sensitive ERP Responses? Brain and Cognition, 147 , 1-12. https://doi.org/10.1016/j.bandc.2020.105671
Anzures, G., Mildort, M., Fennell, E., Bell, C. & Soethe, E. (2022). Race and Early Face-Sensitive Event-Related Potentials in Children and Adults. Journal of Experimental Child Psychology, 214 , 1-21. https://doi.org/10.1016/j.jecp.2021.105287
Anzures, G., Quinn, P.C., Pascalis, O., Slater, A.M. & Lee, K. (2013). Development of Own-Race Biases. Visual Cognition, 21 (9–10), 1165–1182. https://doi.org/10.1080/13506285.2013.821428
Atkinson, A.P. & Adolphs, R. (2011). The Neuropsychology of Face Perception: Beyond Simple Dissociations and Functional Selectivity. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 366 (1571), 1726–1738. https://doi.org/10.1098/rstb.2010.0349
Bar-Haim, Y., Ziv, T., Lamy, D. & Hodes, R.M. (2006). Nature and Nurture in Own-Race Face Processing. Psychological Science, 17 (2), 159–163. https://doi.org/10.1111/j.1467-9280.2006.01679.x
Bigelow, F.J., Clark, G.M., Lum, J.A.G. & Enticott, P.G. (2022). Facial Emotion Processing and Language During Early-to-Middle Childhood Development: An Event Related Potential Study. Developmental Cognitive Neuroscience, 53 , 1-12. https://doi.org/10.1016/j.dcn.2021.101052
Blais, C., Linnell, K.J., Caparos, S. & Estéphan, A. (2021). Cultural Differences in Face Recognition and Potential Underlying Mechanisms. Frontiers in Psychology, 12 , 1-8. https://doi.org/10.3389/fpsyg.2021.627026
Caldara, R., Zhou, X. & Miellet, S. (2010). Putting Culture Under the 'Spotlight' Reveals Universal Information Use for Face Recognition. PloS One, 5 (3), 1-12. https://doi.org/10.1371/journal.pone.0009708
Chua, H.F., Boland, J.E. & Nisbett, R.E. (2005). Cultural Variation in Eye Movements During Scene Perception. Proceedings of the National Academy of Sciences of the United States of America, 102 (35), 12629–12633. https://doi.org/10.1073/pnas.0506162102
Colombatto, C. & McCarthy, G. (2017). The Effects of Face Inversion and Face Race on the P100 ERP. Journal of Cognitive Neuroscience, 29 (4), 664–676. https://doi.org/10.1162/jocn_a_01079
Donchin, E. (1979). Event-Related Brain Potentials: A Tool in the Study of Human Information Processing. In H. Begleiter (Ed.) Evoked Brain Potentials and Behavior (pp. 13-88). Springer.
Duchaine, B. & Yovel, G. (2015). A Revised Neural Framework for Face Processing. Annual Review of Vision Science, 1 (1), 393–416. https://doi.org/10.1146/annurev-vision-082114-035518
Furl, N., Henson, R.N., Friston, K.J. & Calder, A.J. (2013). Top-Down Control of Visual Responses to Fear by the Amygdala. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 33 (44), 17435–17443. https://doi.org/10.1523/jneurosci.2992-13.2013
Gauthier, I., Tarr, M.J., Anderson, A.W., Skudlarski, P. & Gore, J.C. (1999). Activation of the Middle Fusiform “Face Area” Increases with Expertise in Recognizing Novel Objects. Nature Neuroscience, 2 (6), 568–573. https://doi.org/10.1038/9224
Ge, L., Zhang, H., Wang, Z., Quinn, P.C., Pascalis, O., Kelly, D., Slater, A., Tian, J. & Lee, K. (2009). Two Faces of the Other-Race Effect: Recognition and Categorization of Caucasian and Chinese faces. Perception, 38 (8), 1199–1210. https://doi.org/10.1068/p6136
Gobbini, M.I. & Haxby, J.V. (2007). Neural Systems for Recognition of Familiar Faces. Neuropsychologia, 45 (1), 32–41. https://doi.org/10.1016/j.neuropsychologia.2006.04.015
Haist, F. & Anzures, G. (2017). Functional Development of the Brain's Face - Processing System. Wiley Interdisciplinary Reviews. Cognitive Science, 8 (1 – 2), 1-14. https://doi.org/10.1002/wcs.1423
Haxby, J.V., Hoffman, E.A., & Gobbini, M.I. (2000). The Distributed Human Neural System for Face Perception. Trends in Cognitive Sciences, 4 (6), 223–233. https://doi.org/10.1016/s1364-6613(00)01482-0
Herzmann, G. (2016). Increased N250 Amplitudes for Other-Race Faces Reflect More Effortful Processing at the Individual Level. International Journal of Psychophysiology, 105 , 57–65. https://doi.org/10.1016/j.ijpsycho.2016.05.001
Herzmann, G., Bird, C.W., Freeman, M. & Curran, T. (2013). Effects of Oxytocin on Behavioral and ERP Measures of Recognition Memory for Own-Race and Other-Race Faces in Women and Men. Psychoneuroendocrinology, 38 (10), 2140–2151. https://doi.org/10.1016/j.psyneuen.2013.04.002
Herzmann, G., Minor, G. & Adkins, M. (2017). Neural Correlates of Memory Encoding and Recognition for Own-Race and Other-Race Faces in an Associative-Memory Task. Brain Research, 1655 , 194–203. https://doi.org/10.1016/j.brainres.2016.10.028
Herzmann, G., Minor, G. & Curran, T. (2018). Neural Evidence for the Contribution of Holistic Processing but not Attention Allocation to the Other-Race Effect on Face Memory. Cognitive, Affective & Behavioral Neuroscience, 18 (5), 1015–1033. https://doi.org/10.3758/s13415-018-0619-z
Hugenberg, K., Miller, J. & Claypool, H.M. (2007). Categorization and Individuation in the Cross-Race Recognition Deficit: Toward a Solution to An Insidious Problem. Journal of Experimental Social Psychology, 43 (2), 334–340. https://doi.org/10.1016/j.jesp.2006.02.010
Hugenberg, K., Young, S.G., Bernstein, M.J. & Sacco, D.F. (2010). The Categorization-Individuation Model: An Integrative Account of the Other-Race Recognition Deficit. Psychological Review, 117 (4), 1168–1187. https://doi.org/10.1037/a0020463
Kacin, M. & Herzmann, G. (2021). Facial Expressions of Anger Improve Neural Correlates of Memory Retrieval but not Encoding of Only Same-Race Faces. Neuropsychologia, 159 , 1-13. https://doi.org/10.1016/j.neuropsychologia.2021.107915
Kanwisher, N., McDermott, J. & Chun, M. M. (1997). The Fusiform Face Area: A Module in Human Extrastriate Cortex Specialized for Face Perception. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 17 (11), 4302–4311. https://doi.org/10.1523/jneurosci.17-11-04302.1997
Kanwisher, N. & Yovel, G. (2006). The Fusiform Face Area: A Cortical Region Specialized for the Perception of Faces. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 361 (1476), 2109–2128. https://doi.org/10.1098/rstb.2006.1934
Karimi- Rouzbahani, H., Ramezani, F., Woolgar, A., Rich, A. & Ghodrati, M. (2021). Perceptual Difficulty Modulates the Direction of Information Flow in Familiar Face Recognition. NeuroImage, 233 , 1-15. https://doi.org/10.1016/j.neuroimage.2021.117896
Kelly, DJ, Quinn, P.C., Slater, A.M., Lee, K., Ge, L. & Pascalis, O. (2007). The Other-Race Effect Develops During Infancy: Evidence of Perceptual Narrowing. Psychological Science, 18 (12), 1084–1089. https://doi.org/10.1111/j.1467-9280.2007.02029.x
Lebrecht, S., Pierce, L.J., Tarr, M.J. & Tanaka, J.W. (2009). Perceptual Other-Race Training Reduces Implicit Racial Bias. PloS One, 4 (1), 1-7. https://doi.org/10.1371/journal.pone.0004215
Liu, X., Liang, X., Feng, C. & Zhou, G. (2019). Self-Construal Priming Affects Holistic Face Processing and Race Categorization, but not Face Recognition. Frontiers in Psychology, 10 , 1-52. https://doi.org/10.3389/fpsyg.2019.01973
Lopatina, O.L., Komleva, Y.K., Gorina, Y.V., Higashida, H. & Salmina, A.B. (2018). Neurobiological Aspects of Face Recognition: The Role of Oxytocin. Frontiers in Behavioral Neuroscience , 12(195), 1-12. https://doi.org/10.3389/fnbeh.2018.00195
Meissner, C. A., & Brigham, J. C. (2001). Thirty Years of Investigating the Own-Race Bias in Memory for Faces: A Meta-Analytic Review. Psychology, Public Policy, and Law, 7 (1), 3–35. https://doi.org/10.1037/1076-8971.7.1.3
Page, M.J., McKenzie, J.E., Bossuyt, P.M., Boutron, I., Hoffmann, T.C., Mulrow, C.D., Shamseer, L., Tetzlaff, J.M., Akl, E.A., Brennan, S.E., Chou, R., Glanville, J., Grimshaw, J.M., Hróbjartsson, A., Lalu, M.M., Li, T., Loder, E.W., Mayo-Wilson, E., McDonald, S. et al. (2021). The PRISMA 2020 Statement: an updated guideline for reporting systematic reviews. BMJ, 372 (71), 1-9. https://doi.org/10.1136/bmj.n71
Pitcher, D., Duchaine, B. & Walsh, V. (2014). Combined TMS and f MRI Reveal Dissociable Cortical Pathways for Dynamic and Static Face Perception. Current Biology: CB, 24 (17), 2066–2070. https://doi.org/10.1016/j.cub.2014.07.060
Pitcher, D., Pilkington, A., Rauth, L., Baker, C., Kravitz, D.J. & Ungerleider, L.G. (2020). The Human Posterior Superior Temporal Sulcus Samples Visual Space Differently from Other Face-Selective Regions. Cerebral Cortex, 30 (2), 778–785. https://doi.org/10.1093/cercor/bhz125
Pitcher, D. & Ungerleider, L.G. (2021). Evidence for a Third Visual Pathway Specialized for Social Perception. Trends in Cognitive Sciences, 25 (2), 100–110. https://doi.org/10.1016/j.tics.2020.11.006
Proverbio, A.M., Parietti, N. & De Benedetto, F. (2020). No Other Race Effect (ORE) for Infant Face Recognition: A Memory Task. Neuropsychologia, 141 , 1-11. https://doi.org/10.1016/j.neuropsychologia.2020.107439
Prunty, J.E., Jenkins, R., Qarooni, R. & Bindemann, M. (2023). Ingroup and Outgroup Differences in Face Detection. British Journal of Psychology, 114 (S1), 94–111. https://doi.org/10.1111/bjop.12588
Qian, M.K., Quinn, P.C., Heyman, G.D., Pascalis, O., Fu, G. & Lee, K. (2019). A Long ‐ Term Effect of Perceptual Individuation Training on Reducing Implicit Racial Bias in Preschool Children. Child Development, 90 (3), 1-16. https://doi.org/10.1111/cdev.12971
Roth, K.C. & Reynolds, G.D. (2022). Neural Correlates of Subordinate-Level Categorization of Own- and Other-Race Faces in Childhood. Acta Psychologica, 230 , 1-12. https://doi.org/10.1016/j.actpsy.2022.103733
Rugg, M.D. & Curran, T. (2007). Event- Related Potentials and Recognition Memory. Trends in Cognitive Sciences, 11 (6), 251–257. https://doi.org/10.1016/j.tics.2007.04.004
Sangrigoli, S. & de Schonen, S. (2004). Recognition of Own-Race and Other-Race Faces by Three-Month-Old Infants. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 45 (7), 1219–1227. https://doi.org/10.1111/j.1469-7610.2004.00319.x
Saxe, R. & Kanwisher, N. (2003). People Thinking About Thinking People. The Role of the Temporo-Parietal Junction in “Theory of Mind.” NeuroImage, 19 (4), 1835–1842. https://doi.org/10.1016/s1053-8119(03)00230-1
Schwartz, L., Cohen, M., Xu, S., Liu, J. & Yovel, G. (2023). The Social ‐ Encoding Benefit in Face Recognition is Generalized to Other ‐ Race Faces. British Journal of Psychology, 114 (S1), 213 – 229. https://doi.org/10.1111/bjop.12592
Schwartz, L. & Yovel, G. (2019a). Independent Contribution of Perceptual Experience and Social Cognition to Face Recognition. Cognition, 183 , 131–138. https://doi.org/10.1016/j.cognition.2018.11.003
Schwartz, L. & Yovel, G. (2019b). Learning Faces as Concepts Rather Than Percepts Improves Face Recognition. Journal of Experimental Psychology. Learning, Memory, and Cognition, 45 (10), 1733–1747. https://doi.org/10.1037/xlm0000673
Sellal, F. (2022). Anatomical and Neurophysiological Basis of Face Recognition. Revue Neurologique, 178 (7), 649–653. https://doi.org/10.1016/j.neurol.2021.11.002
Shriram, R., Sundhararajan, D.M. & Daimiwal, N. (2012). Brain Connectivity Analysis Methods for Better Understanding of Coupling. International Journal of Computer Science and Information Security, 10 (11), 1-7. https://arxiv.org/ftp/arxiv/papers/1212/1212.3786.pdf
Shoham, A., Kliger, L. & Yovel, G. (2022). Learning Faces as Concepts Improves Face Recognition by Engaging the Social Brain Network. Social Cognitive and Affective Neuroscience, 17 (3), 290–299. https://doi.org/10.1093/scan/nsab096
Sliwinska, M.W., Elson, R., & Pitcher, D. (2020). Dual-Site TMS Demonstrates Causal Functional Connectivity Between the Left and Right Posterior Temporal Sulci During Facial Expression Recognition. Brain Stimulation, 13 (4), 1008–1013. https://doi.org/10.1016/j.brs.2020.04.011
Stelter, M. & Schweinberger, S.R. (2023). Understanding the Mechanisms Underlying the Other - ' Race ' Effect: An Attempt at Integrating Different Perspectives. British Journal of Psychology, 114 (S1), 1 – 9. https://doi.org/10.1111/bjop.12615
Tarr, M.J. & Gauthier, I. (2000). FFA: A flexible Fusiform Area for Subordinate-Level Visual Processing Automated by Expertise. Nature Neuroscience, 3 (8), 764–769. https://doi.org/10.1038/77666
Tüttenberg, S.C. & Wiese, H. (2019). Learning Own- and Other-Race Facial Identities: Testing Implicit Recognition with Event-Related Brain Potentials. Neuropsychologia, 134 , 1-43. https://doi.org/10.1016/j.neuropsychologia.2019.107218
Tüttenberg, S.C. & Wiese, H. (2021). Recognizing Other-Race Faces is More Effortful: The Effect of Individuation Instructions on Encoding-Related ERP Dm Effects. Biological Psychology, 158 , 1-8. https://doi.org/10.1016/j.biopsycho.2020.107992
Walker, P.M., Silvert, L., Hewstone, M. & Nobre, A.C. (2008). Social Contact and Other-Race Face Processing in the Human Brain. Social Cognitive and Affective Neuroscience, 3 (1), 16–25. https://doi.org/10.1093/scan/nsm035
Wiese, H. & Schweinberger, S.R. (2018). Inequality Between Biases in Face Memory: Event-Related Potentials Reveal Dissociable Neural Correlates of Own-Race and Own-Gender Biases. Cortex; A Journal Devoted to the Study of the Nervous System and Behavior, 101 , 119–135. https://doi.org/10.1016/j.cortex.2018.01.016
Wong, H.K., Estudillo, A.J., Stephen, I.D. & Keeble, D.R.T. (2021). The Other-Race Effect and Holistic Processing Across Racial Groups. Scientific Reports, 11 (1), 1-15. https://doi.org/10.1038/s41598-021-87933-1
Wong, H.K., Stephen, I.D., & Keeble, D.R.T. (2020). The Own-Race Bias for Face Recognition in a Multiracial Society. Frontiers in Psychology, 11 (208), 1-8. https://doi.org/10.3389/fpsyg.2020.00208
Yamamoto, H., Kawahara, M., Kret, M. & Tanaka, A. (2020). Cultural Differences in Emoticon Perception: Japanese See the Eyes and Dutch the Mouth of Emoticons. Letters on Evolutionary Behavioral Science, 11 (2), 40–45. https://doi.org/10.5178/lebs.2020.80
Yong, M.H., Lim, X.L. & Schaefer, A. (2020). How do Asians Perceive Caucasian Eyes? Electrophysiological Correlates of Perceiving Racial Differences from the Eyes Region of the Face. Neuroscience Letters, 720 , 1-6. https://doi.org/10.1016/j.neulet.2020.134759
Zhao, M., Cheung, S.-H., Wong, A.C.-N., Rhodes, G., Chan, E.K.S., Chan, W.W.L., & Hayward, W.G. (2014). Processing of Configural and Componential Information in Face-Selective Cortical Areas. Cognitive Neuroscience, 5 (3–4), 160–167. https://doi.org/10.1080/17588928.2014.912207
Zhen, Z., Fang, H. & Liu, J. (2013). The Hierarchical Brain Network for Face Recognition. PloS One, 8 (3), 1-9. https://doi.org/10.1371/journal.pone.0059886
Zhou, X., Burton, A.M. & Jenkins, R. (2021). Two Factors in Face Recognition: Whether You Know the Person's Face and Whether You Share the Person's Race. Perception, 50 (6), 524–539. https://doi.org/10.1177/03010066211014016
1 Product linked to Luis Amigó Catholic University and University of San Buenaventura . “Basic and Applied Neurosciences” Research Group. Funding: none received. Declaration of interests: The authors have no conflicts of interest to declare. Data availability: All relevant data are found in the article.
2 PhD. in Philosophy. PhD candidate in Psychology. Professor at Luis Amigó Catholic University . Email: anyerspn.gomezta@amigo.edu.co
3 Master in Psychology. PhD candidate in Psychology. Professor at University of San Buenaventura. Email: jainer.amezquita@tau.usbmed.edu.co
4 PhD in Psychology. Professor of the Faculty of Psychology. University of San Buenaventura.
Email: david.pineda@usbmed.edu.co